XPS, Chemical Mapping
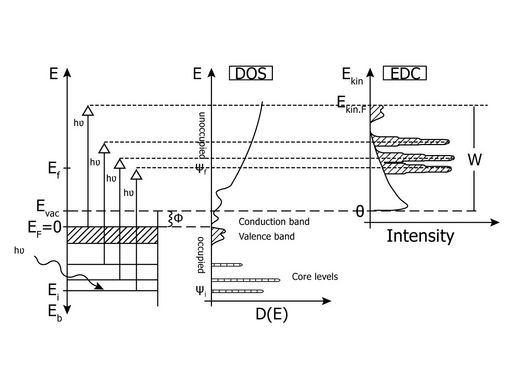
In 1905 Albert Einstein received the Nobel Prize in Physics for his quantum mechanical interpretation of the photoelectric effect. Based on the results of Heinrich Hertz and Max Planck about the nature of light being an electromagnetic wave and about the general existence of discrete energy portions, nowadays named “quantum”, this has been a big step for basic science. At this time nobody knew, that this will evolve into the most important method for non-destructive surface chemical analysis. To reach this understanding the development of energy dispersive electron analyzers had been necessary. Thus it took several decades until Kai Siegbahn developed and experimentally realized the first experiment of this kind in the late 1960s, again resulting in a Nobel Prize in Physics. By excitation of electrons from solid samples using characteristic X-rays and detecting the number of photoelectrons in dependence of their kinetic energies it became possible to use the element specific electron energies to derive the chemical
composition of sample surfaces without destroying them. He named the method Electron Spectroscopy for Chemical Analysis, or in short ESCA. The global success of X-ray Photoelectron Spectroscopy (XPS) is a result of the development of methods for reliable and precise quantification of ESCA data with an elemental detection limit of <1% in the uppermost surface layers. Elemental chemical mapping is possible by energy filtered imaging or mapping of the respective surface.